Welcome to NurseHub’s lesson on States of Matter! This lesson provides video and written material to teach you the basics of solids, liquids, and gases and how they are different from one another. Once you’ve completed all lessons in this section, you can test your knowledge with the section quiz!
Learning Goals for this lesson:
- Describe key features of solids, liquids, gases
- Compare and contrast the properties of solids, liquids, and gases
- Understand the difference in density and molecule motion between solids, liquids, and gases
Key Vocabulary
Let’s review some important words to know for this lesson:
- Solid – a state of matter that has a definite shape and volume because the bonds between the molecules or atoms within the structure are strong
- Liquid – a state of matter that has a definite volume but an undefined shape because there are weak bonds between the molecules or atoms within the substance
- Gas – a state of matter that has an indefinite volume and shape because there are usually no bonds between the molecules or atoms within the substance
- Density – the quantity of matter in a particular unit or amount of volume
- Intermolecular Forces – forces of attraction that bond molecules with other molecules
States of Matter
The states of matter are the different phases or states in which matter can exist. The state of matter of a substance depends on the strength of the intermolecular forces between its particles. The most common states of matter are solid, liquid, and gas.
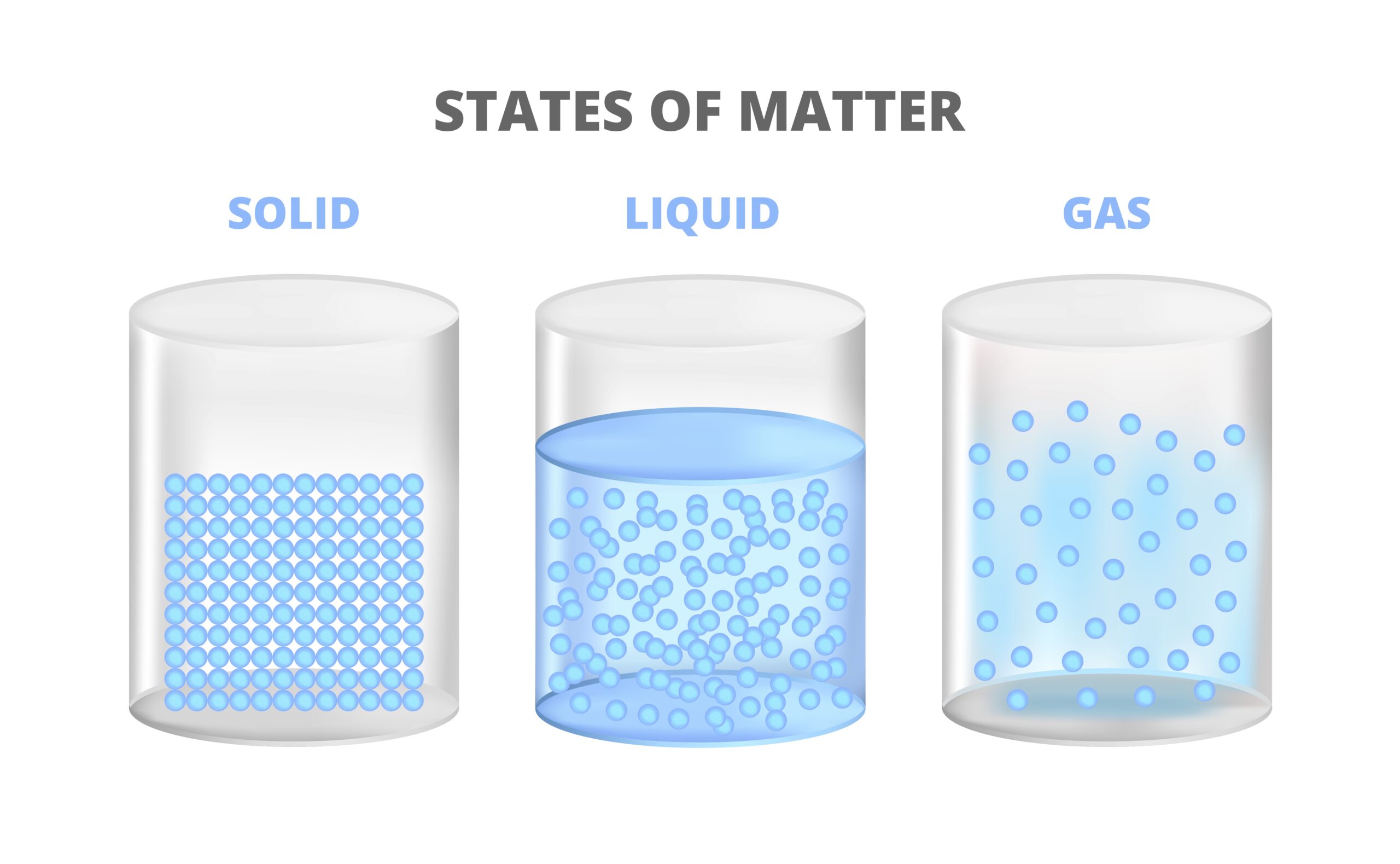
Solids

When matter is in a solid state, the particles are very close together, joined by strong intermolecular forces. This means that solids have a definite shape and volume, the substances have high density, and the particles do not flow around. Their compressibility is low because the particles are already close together. This basically means that they cannot easily be “squished down.” Solids can be crystalline if the particles follow a regular arrangement and amorphous when the particles have an irregular arrangement.
Liquids

Liquid substances are still joined by intermolecular forces, but they are not as strong as in solids. This means that liquids have an indefinite shape and typically take on the shape of their container. Think about the different types of containers we use with water. The water always takes the shape of the specific container we put it into. Liquids have high fluidity, and (in general) their density decreases as temperature increases. Liquids have low compressibility (can’t “squish” it easily) and defined volume (the volume of the liquid doesn’t change).
Gases

Gases have particles that have very weak intermolecular forces, which means that gases have an indefinite (undefined) shape and volume. The molecules in gases tend to move about freely and occupy the entirety of their container. If the container is open, the gas molecules will escape through that opening.
They are also highly compressible (they can be easily “squished down”). This means that they can also expand if the size of the container changes.

Comparing the Density of Solids, Liquids, and Gases
Let’s think about how the properties of the different states of matter impact their density levels. This is important to understand because it helps you tell the difference between the three types and have a better picture of what’s happening to the particles in each one. If you need a quick reminder about what density is, remember it is the measure of how many “things” (particles of mass) are present in a set volume or space.
Use the visual below as a reference when reading through this section:

Solids are typically denser than liquids because their molecules are packed closer together, as you saw in visuals earlier in this lesson.
For a different way to visualize this, think about a group of people standing close together in a small room. If you add more people to the room, they would have to squeeze closer to fit in. The same thing happens with the molecules in a solid. They are tightly packed together and don’t have much room to move around. This is why they are denser than liquids and gases.
Liquids, however, have molecules that are not as tightly packed together. They have more room to move around. This is why liquids can flow and take the shape of their container, while solids have a fixed shape.
So, since the molecules in solids are more closely packed together, they have more mass (particles) within a particular unit of volume than liquids, giving them a higher density. Consider a piece of metal, and the same amount of the metal melted and turned into a liquid. The solid metal will be denser because its molecules are packed together tightly.
Gases are the least dense of the three states of matter because their molecules are the furthest apart from each other. Unlike solids and liquids, gases have a lot of space between their molecules, which makes them less dense.
To understand this concept, think about a balloon. When you blow up a balloon, you are filling it with gas. The gas molecules inside the balloon are spread out and have a lot of space between them. This is why a balloon filled with gas is less heavy than a balloon filled with water (a liquid) or a balloon filled with sand (a solid).
Another way to think about this is by considering the behavior of molecules in a gas. Gas molecules move freely in all directions, bouncing off one another and the walls of their container. The more space there is between molecules, the more room they have to move around, resulting in a lower density.
To sum it all up – gases have the lowest density because their molecules are widely spaced apart, liquids have a higher density than gases because their molecules are closer together, and solids have the highest density because their molecules are tightly packed together.
NurseHub Note:
Unlike most solids, ice is less dense than water. This is because ice acquires a tetrahedral arrangement of its molecules, where each molecule is bonded to four neighboring molecules. This arrangement creates a lattice structure in which the molecules are located farther apart than in liquid water. This special structure is driven by the unique nature of the hydrogen bonds between water molecules. This allows ice to float on water. The structure of the tetrahedral arrangement of ice is shown in the figure below.

Comparing the Movement of Molecules in Solids, Liquids, and Gases
Use the visual below as a reference when reading through this section:

In a solid, the molecules are tightly packed and have a fixed position. They can vibrate in place but cannot move around freely like in a liquid or gas. This is why solids have a fixed shape and volume.
In a liquid, the molecules are still packed closely together, but they have more room to move around than in a solid. This is why liquids can flow and take the shape of their container. The molecules in a liquid can slide past one another, but they still stick together somewhat, which gives liquids a definite volume.
The molecules are much more spread out in a gas than in a solid or liquid. They move around freely and bounce off each other and the walls of their container. This is why gases can expand and fill any container they are in. The molecules in a gas have very little attraction to each other (so, fewer intermolecular forces), which is why gases have no definite shape or volume.
The main differences in molecular movement between solids, liquids, and gases come down to the amount of space between the molecules and how closely they are packed together within a defined space (volume). In a solid, the molecules are tightly packed and vibrate. In a liquid, the molecules can move and slide past one another, but they still stick together somewhat. In a gas, the molecules move around freely and bounce off each other and the walls of their container because they are widely spaced apart.
Let’s Review!
As a quick review, the general characteristics of solids, liquids, and gases are shown in the table below:

Great work! Remember – you can review this lesson as many times as you need. Once you’re ready, head over to the next lesson, where you’ll learn about the processes that occur when substances change from one state of matter to another!

